“They have managed to create what appears to be the goose that really does lay golden eggs!”
That was how UK surgeon Martin Birchall described it to BBC News. The goose in this case is a 3D bioprinter, and the golden eggs are the human sized tissues that the bioprinter successfully constructed. This breakthrough for the field of tissue engineering was reported on Monday in Nature Biotechnology by a research team led by Anthony Atala, director of Wake Forest Institute for Regenerative Medicine.
Bioprinting: yes, it’s actually a thing
To some, printing human body parts may sound like a far-fetched story torn from a science fiction novel but, in reality, development of bioprinters has been underway for a number of years. The bioprinting process isn’t all that different from an inkjet printer except a mixture of gelatin, or hydrogel, and living cells is used as the “ink” to incrementally build a defined biological structure.
As amazing as this technology is, it has met some limitations. Printing cells into 2D shapes and small 3D structures is doable but a lack of structural stability limits building more complex, human-scale tissues. Also, because oxygen and nutrients can only diffuse about 0.004 inches through living tissue, the cells located inside a large bioprinted structure have trouble with long-term survival (check out yesterday’s blog for a mind-blowning story about one lab’s use of cotton candy to deal with this diffusion issue). These challenges have to be overcome before 3D bioprinting can be used for the repair and replacement of human-sized tissues and organs.
Enter ITOP
That’s where the Atala team’s Integrated Tissue-Organ Printer (ITOP), developed over ten years, comes into the picture. For better structural stability, the printer is configured to deliver the cell/hydrogel “bio-ink” within a stronger type of gel, with the fancy name Pluronic F127, that helps the printed cells maintain their shape during the printing process. Afterward, the Pluronic F127 scaffolding mold is simply washed away from the bioprinted tissue.
To ensure adequate oxygen diffusion into the bioprinted tissue, a biodegradable polymer, PCL, is dispensed from the printer at regular intervals; this creates microchannels as stand-ins for blood vessels to help oxygen and nutrients readily reach the interior areas of the tissues. As a bonus, PCL takes about 2 years to biodegrade, providing long term stability.
To prove these innovations of the ITOP actually work, the team built three different tissues: a jawbone fragment, an ear, and muscle.
The making of a jawbone
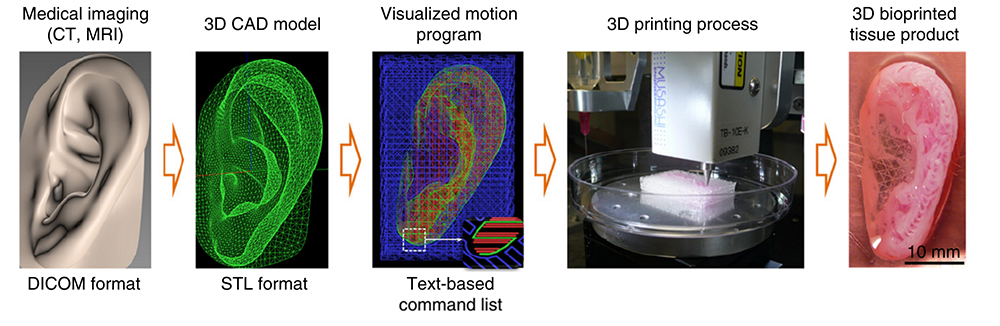
For reconstruction of the jawbone, a 3D computer model was generated from actual CT scan data of a human jaw with a missing piece of bone – something that might be seen in a traumatic injury to a combat soldier (the work is funded in part by the Armed Forces Institute for Regenerative Medicine). That data was fed into the ITOP with coordinates of the precise printing pattern necessary to rebuild the shape of the jaw fragment. In this case, printing was carried out using human amniotic fluid stem cells (AFSC). With the right cues, these stem cells readily specialize into osteogenic, or bone-forming, cells.
Sure enough, 28 days after being cultured in liquid nutrients containing bone-promoting factors, the surface of the bioprinted human jaw showed calcium deposits, the tell-tale signs of bone formation. How would bioprinted bone fare in a living mammal? To find out, the researchers transplanted small discs of AFSC fabricated bone into a bone defect in mice. After 5 months, the transplanted bone was thriving with plenty of blood vessels and no necrosis, or cell death, inside the bone.
The implications of this bone study are pretty cool. In an interview with BBC news, Atala envisions a not so distant future clinical scenario:
“We’d bring the patient in, do the imaging and then we would take the imaging data and transfer it through our software to drive the printer to create a piece of jawbone that would fit precisely in the patient.”
Vincent van Gogh could have used this technology
But the possibilities don’t end with bone. Next, the team tested the ITOP’s talents at building the complex shapes of the outer human ear. In this case, the printer was loaded up with cartilage-producing cells called chondrocytes. The authors posted a fascinating video of the ear bioprinting process in their online publication.
Image: Wake Forest, Nature Biotechnology.
After five weeks in liquid nutrients, a matrix of cartilage had grown throughout the ear. And to look at tissue growth in an animal, the ear was implanted under the skin of the mice. A couple of months after implantation, even more cartilage had formed and the shape of the ear was intact.
But wait there’s more: printing skeletal muscle
Since both the jaw bone and ear cartilage represent hard tissues, the team sought to reconstruct muscle, a soft tissue, with the ITOP. Muscle-forming cells, or myoblasts, were printed to mimic the muscle fiber bundles seen in native skeletal muscle. After growing a week in the lab under conditions that stimulate muscle cell formation, the muscle-like fibers were implanted into rats. Two week after implantation, the bioprinted muscle had not only grown into well-organized muscle fibers, they also were functional in that they were responsive to electrical stimulation.
3D bioprinting: getting closer to reality
Atala is the first to admit that a lot more testing is needed to safely bring this technology into a clinical setting for human use. But as he states in a Wake Forest press release, the ITOP brings 3D bioprinting a step closer to reality:
“This novel tissue and organ printer is an important advance in our quest to make replacement tissue for patients. It can fabricate stable, human-scale tissue of any shape. With further development, this technology could potentially be used to print living tissue and organ structures for surgical implantation.”














![Engineered human esophageal tissue [Credit: The Saban Research Institute].](https://blog.cirm.ca.gov/wp-content/uploads/2014/10/141017092630-large.jpg?w=300)
