The ultimate goal of tissue engineers who work in the regenerative medicine field is to replace damaged or diseased organs with new ones built from stem cells. To accomplish the feat, these researchers are developing new tools and techniques to manipulate and specialize stem cells into three dimensional structures. Some popular methods – which we’ve blogged about often – include the use of bioscaffolds as well as 3D bioprinting . This week, a research team at the Laboratoire Matière et Systèmes Complexes in France has developed an attractive (pun intended!) new tool that uses magnetized stem cells to both manipulate and stimulate the cells into 3D shapes.
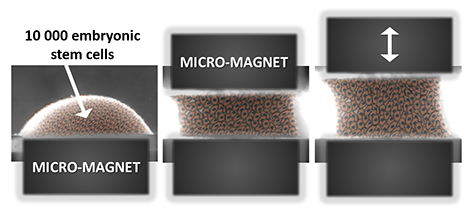
The magnetic stretcher: this all-in-one system can both form and mechanically stimulate an aggregate of magnetized embryonic stem cells. Image: © Claire Wilhelm / Laboratoire Matière et systèmes complexes (CNRS/Université Paris Diderot).
The study, reported on Monday in Nature Communications, used embryonic stem cells which were incubated with magnetic nanoparticles. The cells readily take up the nanoparticles which allowed the scientists to group the individual cells using magnets. But first the team needed to show that the nanoparticles had no negative effects on the cells. Comparing the iron nanoparticle-laden stem cells to iron-free cells showed no difference in the cells’ survival and their ability to divide.
It was also important to make sure the introduction of nanoparticles had no impact on the stem cell’s pluripotency; that is, its ability to maintain its unspecialized state. A visual check of the cells through a microscope showed that they grew together in rounded clumps, a hallmark of undifferentiated, pluripotent cells. In addition, the key genes that bestow pluripotency onto embryonic stem cells were still active after the addition of the nanoparticles.
The stem cells’ ability to mature into various cell types, like heart muscle or nerve, is key to any successful tissue engineering project. So, the next important assessment of these magnetized cells was to make sure their ability to differentiate, or specialize, was still intact. The typical first step to differentiating embryonic stem cells is to form so-called embryoid bodies (EBs), which are 3D groups of pluripotent stem cells which begin differentiating into the three fundamental tissues types: mesoderm (gives rise to muscle, bone, fat), ectoderm (gives rise to nerve, hair, eyes), endoderm (gives rise to intestines, liver). Using a popular technique, called the hanging drop method, the team showed that the presence of the nanoparticles did not negatively affect embryoid body formation.
In fact, the use of magnets to form embryoid bodies provided several advantages over the hanging drop method. The hanging drop technique requires multiple, time-consuming steps and the resulting embryoid bodies tend to be inconsistent in size and shape. Use of the magnets, on the other hand, instantaneously assembled the stem cells into consistently round aggregates. And by precisely adjusting the magnetic force used, the scientists could also vary the size of the embyroid body, which is an important variable to control since the embryoid size can impact its ability to differentiate.
While the magnet used to form the embryoid bodies was kept stable, the researchers included another magnet which they could move. With this setup, the team was able to stretch and shape the group of cells without the need of scaffolds or the need to physically contact the cells. Several previous studies, using flat, 2-dimensional petri dishes, have shown that the stiffness and flexibility of the dish can stimulate gene activity by affecting cell shape. In this study, the researchers found that when the magnet was moved in a cyclical pattern that imitates the rhythm of a heart beat, the embryoid bodies were, if you can believe it, nudged toward a heart muscle fate. A press release by France’s National Center of Scientific Research (CNRS), which funded the study, explained the big picture implications of this new technique:
“This “all-in-one” approach, which makes it possible to build and manipulate tissue within the same system, could thus prove to be a powerful tool both for biophysical studies and tissue engineering.”