Tomorrow, February 28th, is Rare Disease Day. It’s a day to remind ourselves of the millions of people, and their families, struggling with these diseases. These conditions are also called orphan diseases because, in many cases, drug companies were not interested in adopting them to develop treatments.
Here at the California Institute for Regenerative Medicine (CIRM), we understand the importance of funding research that impacts not just the most common diseases. In fact, 50% of all the projects we fund target a rare disease or condition such as: Retinitis pigmentosa, Sickle cell disease, Huntington’s disease, and Duchenne Muscular Dystrophy.
Over the years, CIRM has invested millions of dollars in helping children born with severe combined immunodeficiency (SCID), including $12 million to test a newly designed therapy in a clinical trial at UC San Francisco.
Children born with SCID have no functioning immune system so even a simple infection can prove life-threatening or fatal. We recently shared an update from one of the young patients in the trial.
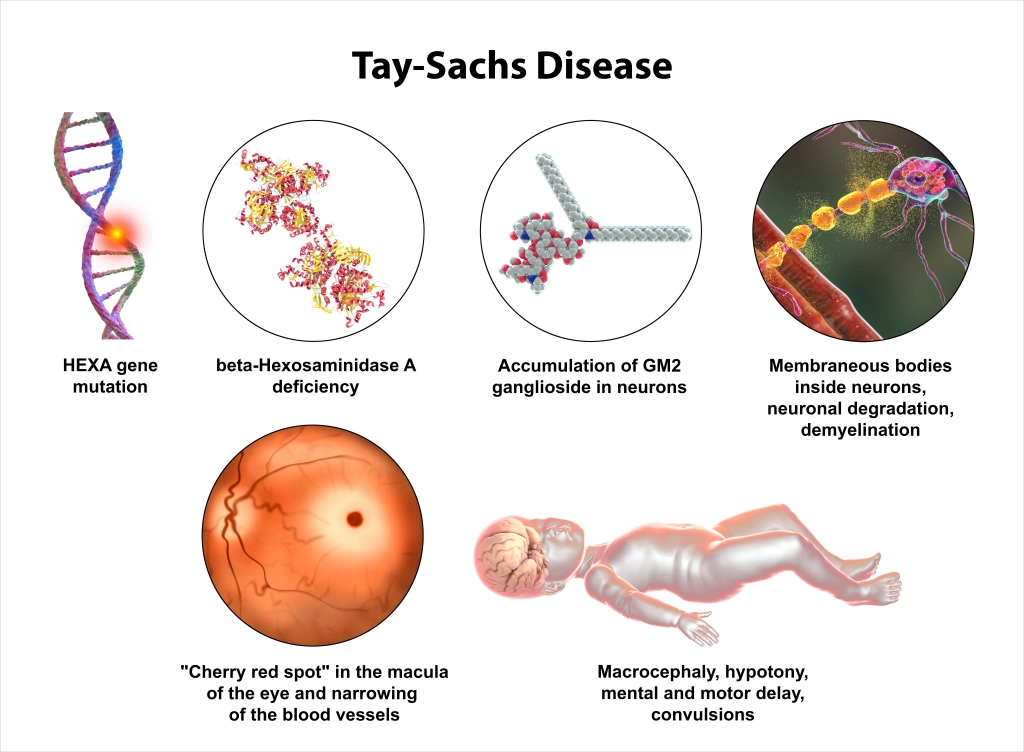
Additionally, last December, the CIRM governing Board awarded $4,048,253 to Dr. Joseph Anderson and his team at UC Davis to develop a blood stem cell gene therapy for the treatment of Tay-Sachs disease.
Tay-Sachs disease is a rare genetic disorder where a deficiency in the Hex A gene results in excessive accumulation of certain fats in the brain and nerve cells and causes progressive dysfunction.
There are several forms of Tay-Sachs disease, including an infant, juvenile, and adult forms. Over a hundred mutations in the disease-causing Hex A gene have been identified that result in enzyme disfunction. There are currently no effective therapies or cures for Tay-Sachs.
The irony of rare diseases is that a lot of people have them. The total number of Americans living with a rare disease is estimated at between 25-30 million. Two-thirds of these patients are children.
Right now, individual disease programs tend to try individual approaches to developing a treatment, which is time consuming and expensive. That’s why this past summer, CIRM signed a Memorandum of Understanding (MOU) with the Foundation for the National Institutes of Health (FNIH) to join the Bespoke Gene Therapy Consortium (BGTC).
BGTC is a public-private partnership, managed by FNIH, that brings together the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA), and multiple public and private sector organizations to streamline the development and delivery of gene therapies for rare diseases.
“At CIRM we have funded several projects using gene therapy to help treat, and even cure, people with rare diseases such as severe combined immunodeficiency,” says Dr. Maria T. Millan, the President and CEO of CIRM. “But even an agency with our resources can only do so much. This agreement with the Bespoke Gene Therapy Consortium will enable us to be part of a bigger partnership, one that can advance the field, overcome obstacles and lead to breakthroughs for many rare diseases.”
CIRM is proud to fund and spread awareness of rare diseases and invites you to watch this video about how they affect families around the world.