
An unregulated stem cell treatment in 2001 led to tumor growth in the (A) brain stem and (B) spinal cord of the patient four years later. (Fig 1. PLoS Med. 2009 Feb 17;6(2):e1000029)
A real stem cell tourism story
Back in 2001, an Israeli boy suffering from Ataxia Telangiectasia, a genetic brain disease that affects movement, traveled to Russia for an unregulated stem cell treatment. His brain and spinal cord were injected with fetal stem cells though the exact composition of those cells was not known. Four years later, the boy complained of headaches and his doctors back home found tumors in his brain and spinal cord.
Stem cells: a double-edged sword
As the BBC and many other news outlets reported in 2009, a Plos Medicine report eventually confirmed the tumor cells originated from the donor stem cells. And here lies a double-edged sword of stem cell-based therapies. On one side, stem cells hold great promise to repair diseased or damaged tissue because they can morph, or differentiate, into a wide range of cell types.
But on the other side, they have the capacity to remain unspecialized and continually self-renew.This is great for producing enough cells to treat many people. Researchers try to make sure only more mature cells are transplanted, but if any of these propagating, undifferentiated cells get carried along with a stem cell-based treatment, there’s a risk of introducing uncontrolled cell growth and cancers instead of remedies. Human pluripotent stem cells (hPSCs), which can form almost any cell type found in our body, are believed to be especially susceptible to this dangerous potential side effect.
Reporting this week in the journal, eLife, CIRM-funded researchers at UCSD found a way to dodge the risk of tumor growth by identifying a unique, alternate stem cell type that could be applied to kidney disease. To find this cell type, the research team focused on cells that were a bit further along a differentiation path compared to unspecialized hPSCs.
Repeat after me: endoderm, ectoderm, mesoderm
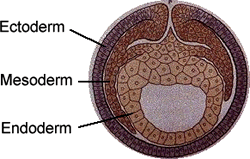
In the earliest stages of embryo development, three germ layers form. (image: Internet Science Room)
To explain, let’s take a brief detour into developmental biology. In the very early stages of specialization, the cells of the embryo form the three germ layers: ectoderm, endoderm and mesoderm. Each layer gives rise to specific set of cells and tissues. Endoderm forms, to just name a few, the lungs, intestines and pancreas; ectoderm develops into skin, the brain and spinal cord; mesoderm forms blood, muscle, bone and kidneys. Within each germ layer lie progenitor stem cells, that maintain the capacity to self-renew and can also differentiate into the adult cells formed by that germ layer.
Finding a mesoderm progenitor
While methods for growing ectoderm and endoderm progenitor stem cells from hPSCs had been previously developed, few, if any, labs had done the same for mesoderm. So the UCSD team systematically tested thousands of combinations of nutrients and chemicals for both growing and differentiating hPSCs into mesoderm. Using this approach, they successfully tracked down a recipe that gave rise to mesoderm progenitor cells with the potential to multiply and grow in population yet lacking the ability to form tumors when transplanted into mice.

Color tagged surface proteins indicate a kidney fate for activated mesodermal progenitors (Fig 7c Kumar et al. eLife 2015;4:e08413)
The research team planned to work out the various conditions to specialize the progenitor cells into a wide range of mesoderm tissues. But to their surprise, when triggered to differentiate, the progenitors only gave rise to cells of the kidney. This very limited specialization is actually desired for clinical applications since purity of cell therapies is a requirement for testing in humans.
Our kidneys thank you
Putting it all together, the team has identified a cell source with unlimited self renewal capacity that can differentiate into a very specific cell type and doesn’t carry a risk of tumor formation when transplanted. These qualities make the mesoderm progenitor cell an exciting tool for developing future kidney repair or replacement treatments. And as Dr. Karl Willert, senior author and associate professor at UC San Diego, states in a UCSD press release, there is also reason to be excited about near-term applications:
“Our cells can serve as building blocks to generate kidneys that may one day be suitable for cell replacement and transplantation. I think such a therapeutic application is still a few years in the future, but engineered kidney tissue can serve as a powerful model system to study how the human kidney interacts with and filters drugs. Such an application would be of tremendous value to the pharmaceutical industry.”