Just 31 years old, Richard Lajara thought he was going to die.

Richard Lajara, the 4th participant in Geron’s stem cell-based clinical trial for spinal cord injury.
On September 9, 2011 he slipped on some rocks at a popular swimming hole and was swept down a waterfall headfirst into a shallow, rocky pool of water. Though he survived, the fall left him paralyzed from the waist down due to a severed spinal cord.
Patient Number Four
At that same time period, Geron Inc. had launched a clinical trial CIRM helped fund testing the safety of a stem cell-based therapy for spinal cord injury (SCI). It was the world’s first trial using cells derived from human embryonic stem cells and Lajara was an eligible candidate. Speaking to CIRM’s governing Board this past summer for a Spotlight on Disease seminar, he recalled his decision to participate:
“When I participated with the Geron study, I was honored to be a part of it. It was groundbreaking and the decision was pretty easy. I understood that it was very early on and I wasn’t looking for any improvement but laying the foundation [for future trials].”
A few months after his treatment, Geron discontinued the trial for business reasons. Lajara was devastated and felt let down. But this year the therapy got back on track with the announcement in June by Asterias Biotherapeutics that they had treated their first spinal cord injury patient after having purchased the stem cell assets of Geron.
Getting Hope Back on Track
Dr. Jane Lebkowski, Asterias’ President of R&D and Chief Scientific Officer, also spoke at the Spotlight on Disease seminar to provide an overview and update on the company’s clinical trial. A video recording of Lebkowski’s and Lajara’s presentations is now available on our web site and posted here:
As Dr. Lebkowski explains in the video, Asterias didn’t have to start from scratch. The Geron study data showed the therapy was well tolerated and didn’t cause any severe safety issues. In that trial, five people (including Richard Lajara) with injuries in their back received an injection of two million stem cell-derived oligodendrocyte progenitor cells into the site of spinal cord damage. The two million-cell dose was not expected to show any effect but was focused on ensuring the therapy was safe.
Oligodendrocyte Precursors: Spinal Cord Healers
As the former Chief Scientific Officer at Geron, Lebkowski spoke first hand about why the oligodendrocyte precursor was the cell of choice for the clinical trial. Previous animal studies showed that oligodendrocyte progenitors, a cell type normally found in the spinal cord, have several properties that make them ideal cells for treating SCI: first, they help stimulate the growth of damaged neurons, the cell type responsible for transmitting electrical signals from the brain to the limbs.
Second, the oligodendrocytes produce myelin, a protein that acts as an insulator of neurons, very much like the plastic covering on a wire. In many spinal cord injuries, the nerves are still intact but lose their myelin insulation and their ability to send signals. Third, the oligodendrocytes release other proteins that help reduce the size of cysts that often form at the injury site and damage neurons. In preclinical experiments, these properties of oligodendrocyte progenitors improved limb movement in spinal cord-severed rodents.
Together, the preclinical animal studies and the safety data from the Geron clinical trial helped Asterias win approval from the Food and Drug Administration (FDA) to start their current trial, also funded by CIRM, this time treating patients with neck injuries instead of back injuries.
The Asterias trial is a dose escalation study with the first group of three patients again receiving two million cells. The trial was designed such that if this dose shows a good safety profile in the neck, as it did in the Geron trial in the back, then the next cohort of five patients will receive 10 million cells. In fact, Asterias reported in August that the lower dose was not only safe but also showed some encouraging results in one of the patients. And just two days ago Asterias announced their data monitoring committee recommended to begin enrolling patients for the 10 million cell dose. If all continues to go well with safety, the dose will be escalated to 20 million cells in the third cohort of five patients. While two million cells was a very low safety dose, Asterias anticipates seeing some benefit from the 10 and 20 million cell doses.
Changing Lives by Increasing Independence
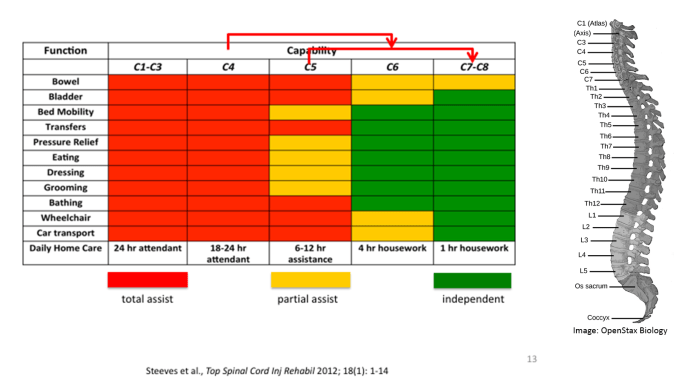
Does Lebkowski’s team expect the patients to stand up out of their wheelchairs post-treatment? No, but they do hope to see a level of improvement that could dramatically increase quality of life and decrease the level of care needed. Specifically, they are looking to see a so-called “two motor level improvement.” In her talk Lebkowski explained this quantitative measure with the chart below:
“If a patient is a C4 [meaning their abilities are consistent with someone with a spinal cord injury at the fourth cervical, or neck, bone] they will need anywhere from 18 to 24 hours of attendant care for daily living. If we could improve their motor activity such that they become a C6, that is just two motor levels, what you can see is independence tremendously increases and we go from 18 to 24 hour attendant care to having attendant care for about four hours of housework.”
It’s so exciting the field is at a point in time that scientists like Dr. Lebkowski are discussing real stem cell-based clinical trials that are underway in real patients who could achieve real improvements in their lives that otherwise would not be possible.
And we have people like Richard Lajara to thank. I think Dr. Oswald Stewart, the Board’s spinal cord injury patient advocate, summed it up well when speaking to Lajara at the meeting:
“Science and discovery and translation [into therapies] doesn’t happen without people like you who are willing to put yourselves on the line to move things forward. Thank you for being in that first round of people testing this new therapy.”
I would like to join the trial I’m a quadriplegic C5 I live in Philadelphia PA 215 744 2524
Hi Abigail – Thanks for reaching out to us. To see if you qualify for this trial, please visit this page: http://www.spinal-cord-injury-study.com/ One thing I didn’t mention in the blog is that the trial is limited to people who have been injured 14-30 days prior to treatment.
Here’s another trial that’s currently recruiting: https://www.sciresearchstudy.com/
And to search for other trials for spinal cord injury, visit: http://www.clinicaltrials.gov.
For information about stem cell treatments in general, here’s a great resource: http://www.closerlookatstemcells.org/stem-cells-and-medicine/
Best Wishes -Todd
What do you think of the considerations made in this blog? http://stemcellassays.com/2015/05/dead-arrival/
Hello douglasmacarthur, thanks for your question: we would just emphasize that the knowledge gained from these pioneering studies is very valuable. We believe that this learning will enable companies to move the concepts forward for patients.
Hello, My name is Steve Arreola, from Dallas, Texas. My sister is C1-3 since December 2017 1yr and 2mths. Are there any trials that would be willing to try?… I
I’ve read that most trials are asking for recently injured patients. Please help!
Hi Steve, unfortunately what you have heard is true. Most trials are looking for people with very recent injuries, usually within the last month. I think the idea is that these kinds of injuries are most likely to respond to stem cells and that once we have found a way to help these patients we can learn how to help others, like your sister, with longer-term injuries.