The FDA has granted Regenerative Medicine Advanced Therapy (RMAT) designation for a project focused on vision loss that was funded in part by the California Institute for Regenerative Medicine (CIRM). This designation is given to highly promising regenerative medicine therapies that treat serious or life-threatening diseases with the goal of accelerating the path to FDA approval and widespread use.
The designation went to a promising therapy for dry age-related macular degeneration (AMD) being developed by Luxa Biotechnology.
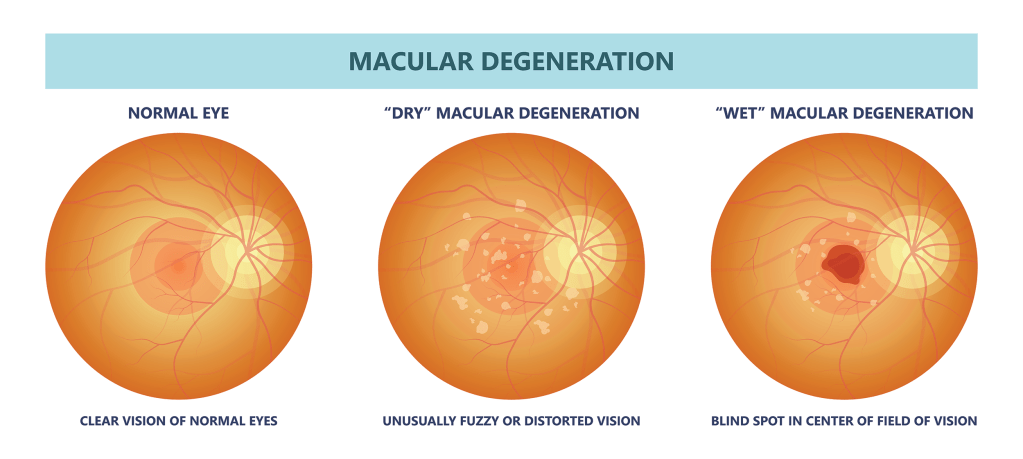
Luxa’s website describes dry AMD as follows: The back of the eye is lined by a layer called the retina, a thin layer of nerve tissue that acts like the film inside a camera. In the middle of the retina is the macula, a concentration of retinal pigment epithelial (RPE) and photoreceptor cells that detect light to initiate vision. Dry AMD is an eye disease caused by RPE and photoreceptor cell degeneration in the macula.
Nearly 20 million people in the U.S. experience dry AMD, which occurs when the macular cells in the eye deteriorate. The Luxa study implants RPE cells to restore vision.

Researchers at Luxa grow adult RPE stem cells (RPESC) in a lab dish to generate new RPE cells (RPESC-RPE). The new cells are grown for four weeks to produce the progenitor stage RPESC-RPE-4W cell product that is transplanted into the back of a person’s eye.
The company is in the midst of a Phase 1/2a clinical trial in patients with dry AMD. That clinical trial—led by Jeffery Stern, PhD, MD, Luxa’s Chief Medical Officer—is funded in part by a $4 million CIRM grant. Dr. Stern emphasized that the RMAT achievement has been a team effort including CIRM, the NIH National Eye Institute, and the FDA.
Dr. Stern will present data from the Phase 1 trial at the Wills Eye Conference in Philadelphia on March 6, 2025.
“The RMAT designation was granted following the FDA’s review of our compelling preliminary Phase 1/2a clinical data and underscores the transformative potential of RPESC-RPE-4W in addressing the unmet needs of dry AMD patients who have lost or are losing their vision,” said Keith Dionne, PhD, CEO of Luxa. “We look forward to collaborating closely with the FDA to bring this potential paradigm-changing treatment to patients as efficiently as possible, restoring vision and improving the quality of life for millions.”
CIRM has provided more than $200 million in funding for research seeking to better understand vision loss and develop new cell and gene therapies.
“Luxa’s therapy has shown potential in restoring lost vision in patients with dry AMD and improving quality of life for people with this condition that affects millions,” said Joseph Gold, PhD, Senior Director of Clinical Development at CIRM. “CIRM has funded multiple projects for dry AMD, underscoring our commitment to advancing research and treatments for patients with vision loss.”
This is the first CIRM-funded clinical trial for dry AMD to receive RMAT designation.
Written by guest contributor Amy Adams
Oh wow, great new, I wish this could help me regain my vision… I had an accident and damaged my left eye.. the doctor said it’s a scar on the retinal and nothing can’t done to regain my vision… I can only very blurry on the left eye.. my right eye is good, Thank God! 🙏