
When someone thinks of machine learning, the first thing that comes to mind might be the technology used by Netflix or Hulu to suggest new shows based on their viewing history. But what if this technology could be applied towards advancing the field of regenerative medicine?
Thanks to a CIRM funded study, a team of scientists lead by Dr. Todd McDevitt at the Gladstone Institutes have found a way to to use machine learning to control the spacial organization of stem cells, a key process that plays a vital role in organ development. This new understanding of how stem cells organize themselves in 3D is an important step towards being able to create functional and/or personalized organs for research or organ transplants.
“We’ve shown how we can leverage the intrinsic ability of stem cells to organize,” said Dr. McDevitt in a news release from Gladstone Institutes. “This gives us a new way of engineering tissues, rather than a printing approach where you try to physically force cells into a specific configuration.”
In their normal environment, stem cells are able to form patterns as they mature and over time morph into the tissues seen in an adult organism. One type of stem cell, called an induced pluripotent stem cell (iPSC), can become nearly every cell type of the body. In fact, researchers have already found ways to direct iPSCs to become various cell types such as those in the heart or brain.
Unfortunately, attempting to replicate the pattern formation of stem cells as they mature has been challenging. Some have used 3D printing to lay out stem cells in a desired shape, but the cells often migrated away from their initial locations.
In the same news release mentioned above, Ashley Libby, a graduate student at Gladstone and co-first author of this study, said that,
“Despite the importance of organization for functioning tissues, we as scientists have had difficulty creating tissues in a dish with stem cells. Instead of an organized tissue, we often get a disorganized mix of different cell types.”
To solve this problem, the scientists used a computational model to learn how to influence stem cells into forming new arrangements, such as those that might be useful in generating personalized organs.
Previous studies conducted by Dr. McDevitt showed that blocking the expression of two genes, called ROCK1 and CDH1, affected the layout of iPS cells grown in a petri dish.
In this current study, the scientists used CRISPR/Cas9 gene editing (you can read about this technology in more detail here) to block expression of ROCK1 and CDH1 at any time by adding a drug to the iPSCs. This was done to see if they could predict stem cell arrangement based on the alterations made to ROCK1 and CDH1 at different drug doses and time periods.
The team carried out various experiments with different doses and timing. Then, the data was input into a machine-learning program designed to identify patterns, something that could take a human months to identify.
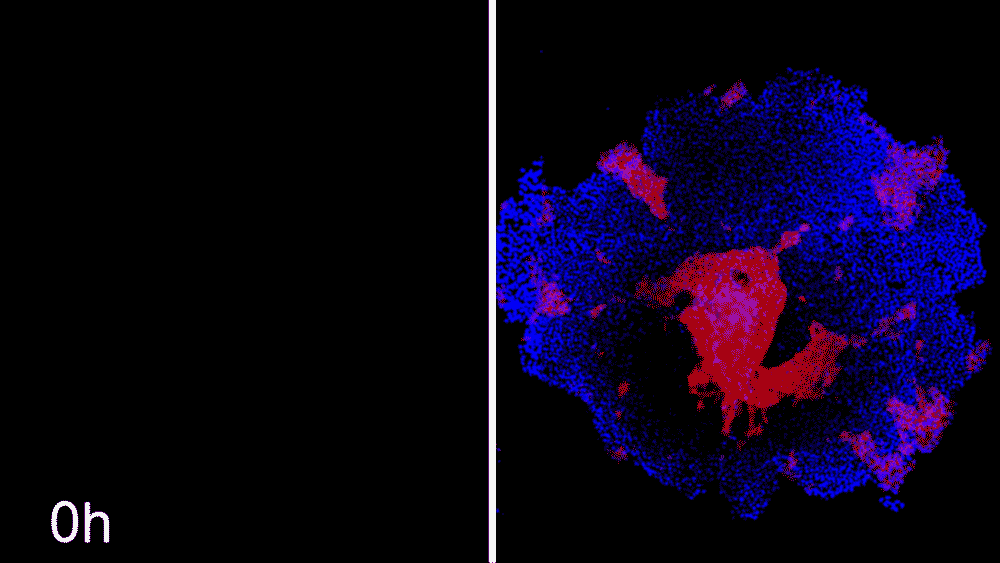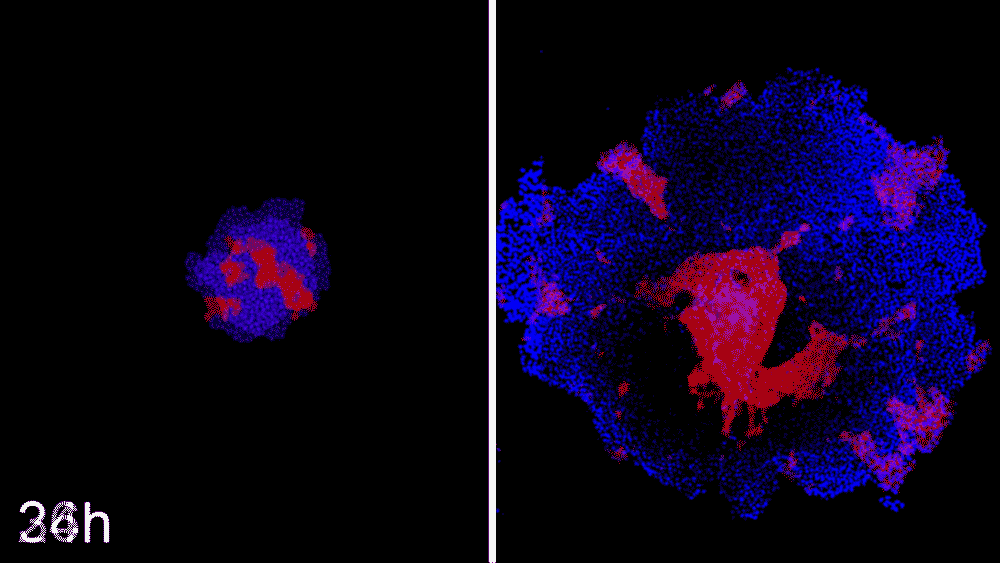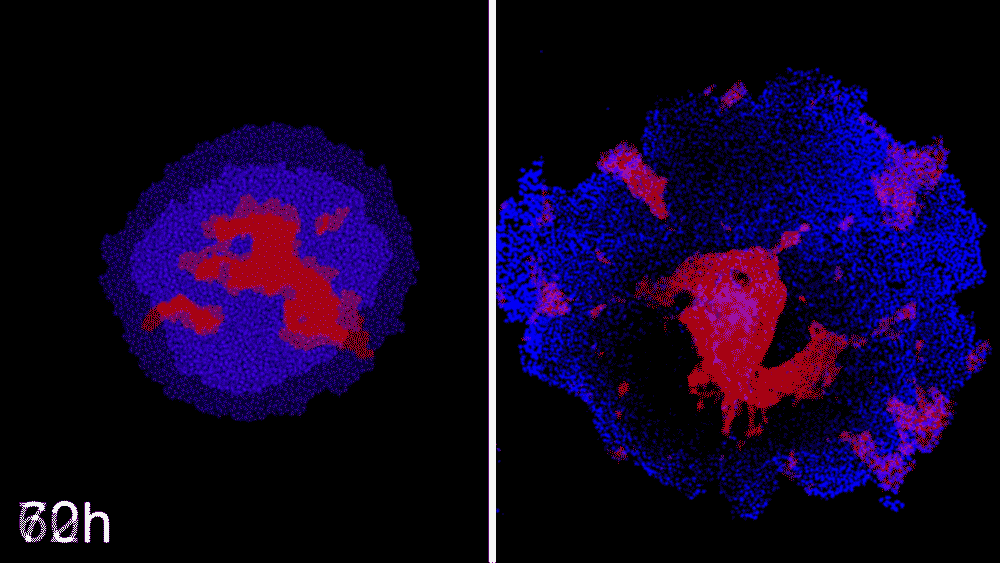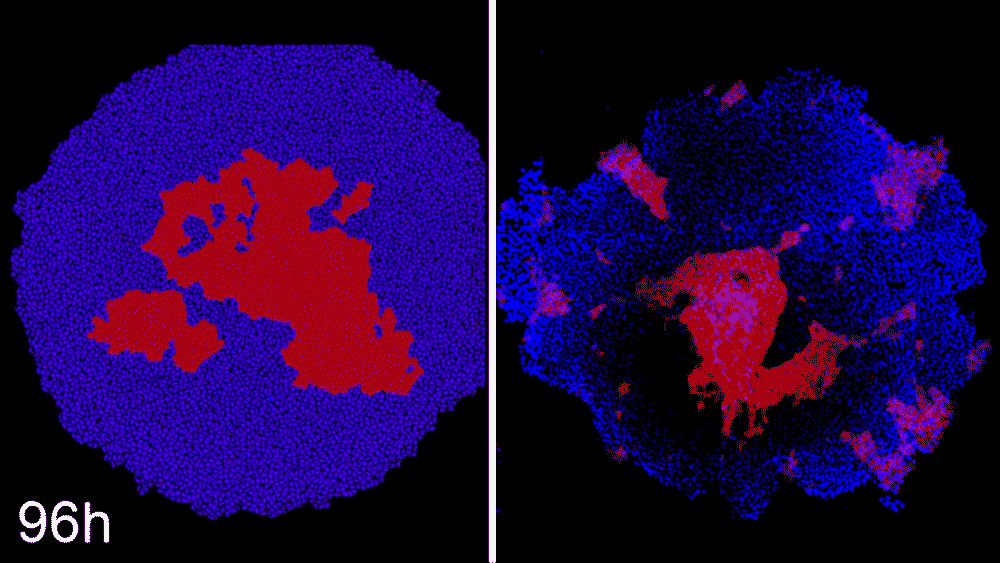
The machine-learning program used the data to predict ways that ROCK1 and CDH1 affect iPSC organization. The scientists then began to see whether the program could compute how to make entirely new patterns, like a bull’s-eye or an island of cells. The team says the results were little short of astounding. Machine learning was able to accurately predict conditions that will cause stem cell colonies to form desired patterns.
The full study was published in the journal Cell Systems.