Neurona Therapeutics has reached a major milestone in the development of its stem cell approach to treating epilepsy. The company recently announced that the first patient has been dosed in a Phase 1/2 clinical trial evaluating NRTX-1001, a cell therapy for adults whose epilepsy affects both sides of the brain and for whom drugs have not been effective.
This same therapy has demonstrated positive results in a trial for people whose epilepsy affects one side of the brain. This new trial is supported by a $14 million award from the California Institute for Regenerative Medicine (CIRM). CIRM has supported this research from its early discovery stage at UCSF to the current first-in-human clinical trials.
Epilepsy causes recurring seizures in the brain and affects more than 3 million people in the United States, including more than 500,000 in California. Although many people with epilepsy benefit from anti-seizure medications, about one-third of patients don’t benefit from those drugs. These people are often unable to work, drive, or carry out many daily tasks.
Current treatments for drug-resistant epilepsy often involve invasive surgeries that destroy brain tissue and carry serious, irreversible risks. Moreover, patients with epilepsy on both sides of the brain are typically not eligible for these surgeries due to the risk of profound and permanent memory loss.
NRTX-1001 is a cell therapy composed of neurons derived from human embryonic stem cells that inhibit excitatory neurons. When injected into the brain, these cells are designed to calm overactive neurons and reduce seizures without damaging healthy brain tissue. In this trial, patients receive a one-time injection of NRTX-1001 into both sides of the brain.
Because this is a phase 1 trial, it is designed primarily to evaluate safety, tolerability, and preliminary efficacy, with follow up assessments for two years after treatment.
Building on Encouraging Results in Unilateral Epilepsy Trial
The new bilateral trial builds on encouraging results from Neurona’s ongoing trial—which observes seizures that originate from one side of the brain—and is also funded and supported by CIRM. Presented earlier this year, those findings showed a 92% reduction in seizures and overall improvements in quality of life. All participants receiving the therapy reported no cognitive decline or cell therapy-related adverse events.
One of those participants was Justin Graves, who in 2007 experienced his first epileptic seizure while working at a SCUBA shop in Louisville, Kentucky, forcing him to give up driving and diving—activities that had been central to his life.

Like roughly 30% of people with epilepsy, Justin’s seizures were drug-resistant, and though lifestyle changes eventually improved his response to medication, he continued to experience debilitating episodes.
After relocating to California, Justin was introduced to Neurona’s clinical trial, which aimed to treat his focal epilepsy using NRTX-1001, which was injected into the seizure-generating region of the brain.
Justin underwent the procedure at UC San Diego in 2023 and began noticing improvements within a month. He no longer suffered from grand mal seizures and experienced only occasional mild episodes known as auras.
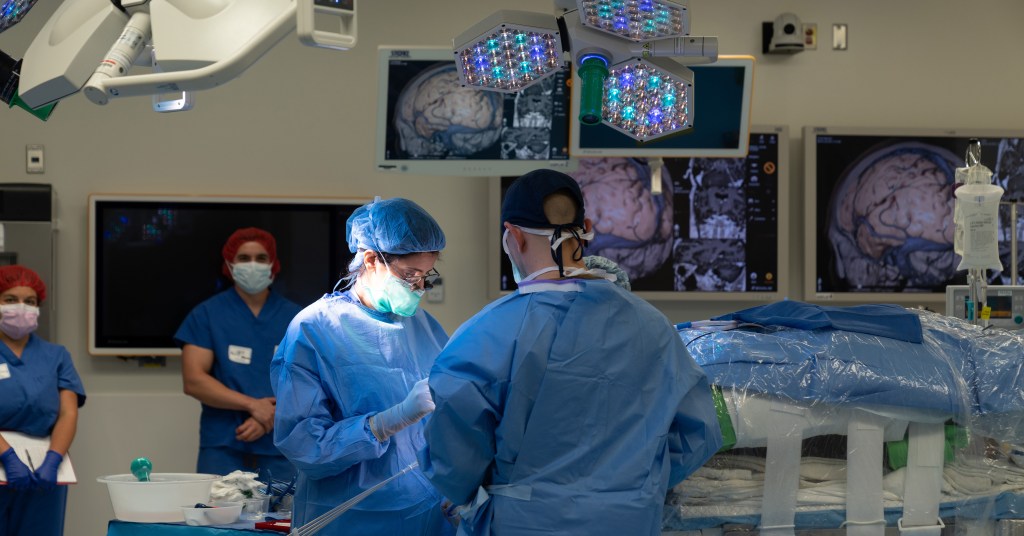
While the Phase 1 trial primarily evaluated the therapy’s safety, early signs of efficacy were encouraging. Unlike traditional epilepsy surgeries that remove or damage brain tissue, this new approach offers a non-destructive alternative, potentially transforming how epilepsy is treated.
Justin said of being an early participant in the unilateral clinical trial, “I love the idea of being able to help not just a few people with some issues, but the entire world.”
What’s Next?
In addition to initiating the bilateral trial, Neurona has enrolled people into an expansion of its unilateral study and plans to present updated data from both trials at the American Epilepsy Society’s Annual Meeting in December 2025.
“We are sincerely grateful for the continued support and guidance from CIRM,” said Cory R. Nicholas, PhD, Neurona’s Chief Executive Officer and Co-Founder. “NRTX-1001’s progress is a testament to CIRM’s vision and commitment to funding innovative science from bench to bedside. With this new award, we now have the opportunity to expand potential indications for NRTX-1001 to include the drug-refractory bilateral TLE population, which is of highest unmet medical need in epilepsy.”
If successful, data from these trials—along with an upcoming Phase 3 study—could support future FDA approval of NRTX-1001 for drug-resistant epilepsy, offering a potential treatment for patients and families affected by this life-altering condition.