Researchers learn a ton about the biological function of cells by studying them in vitro; that is, outside the body in a petri dish. But inside the body, or in vivo, cells respond to surrounding proteins and other cells that may be missing in an in vitro experiment. Important insights waiting to be revealed can easily be overlooked if a cell isn’t analyzed in the right context.
That’s the lesson learned from a recent study in Science looking at the induced pluripotent stem (iPS) cell process of reprogramming adult skin cells into an embryonic stem cell-like state. By examining this technique in laboratory mice, a research team at the Spanish National Cancer Research Centre (CNIO) showed that, compared to isolated cells in vitro, the efficiency of in vivo cellular reprogramming in the mice is boosted by nearby damaged cells. So injured cells appear to provide a signal to help kick start the regenerative process.
(Watch this video for a quick recap of the report or read on for a few more details)
The history of iPS cells in 30 seconds
But let’s a take a quick step back. Actually ten years back. That’s when Shinya Yamanaka discovered that the insertion of just four genes – let’s call them the Yamanaka genes – into adult skin cells in vitro can wipe their identities clean allowing them to be specialized into virtually any cell type. While this ground breaking work led to a Nobel Prize, the efficiency of the method was very low. Research in the past couple of years has shown in vivo reprogramming is also possible but also at a low efficiency.
So what’s behind the low efficiency? Some culprits include tumor suppressor proteins (which act like kill switches in cells to prevent them from turning into cancer) like p53 and INK4. Blocking the activity of either protein increases the efficiency of in vitro reprogramming. But a funny thing happened in the current study when the researchers did the same thing in vivo. They injected mouse skin cells with the Yamanaka reprogramming genes into mice lacking the p53 gene or the INK4 gene or into control mice with both genes intact. Compared to the control mice, in vivo reprogramming efficiency was higher in the mice missing the p53 gene, as you’d expect based on the in vitro results described above. But in mice without the INK4 gene, the efficiency was actually lower than the control. That’s the exact opposite of the in vitro case in which blocking INK4 increases reprogramming efficiency.
Who knew? Cell slow down stimulates the iPS process in surrounding cells
To investigate this baffling result, the team focused on the fact that INK4 plays a role in cell senescence. When cells get old or damaged they become senescent; that is, they stop dividing and release proteins that cause inflammation. Now, it turns out that in vivo, the Yamanaka genes not only drive reprogramming but they also lead to a lot of damage to surrounding cells causing them to senesce.
And herein lies the answer. The in vivo reprogramming efficiency appears to depend on surrounding cells becoming senescent. Cells in the mice lacking INK4 don’t senesce, and the resulting reprogramming efficiency is low. But in mice lacking p53, the team observed lots of senescent cells along with increased reprogramming efficiency.
By studying the various inflammation-causing proteins that senescent cells release, the team zeroed in on a protein called IL-6 as the connection between reprogramming and senescence. When IL-6 was blocked, in vivo reprogramming efficiency dropped. The team also mimicked these results in vitro. When damaged cells were present while reprogramming cells in the same petri dish, efficiency increased. And when IL-6 was removed from the nutrients the cells were grown in, in vitro reprogramming efficiency decreased.
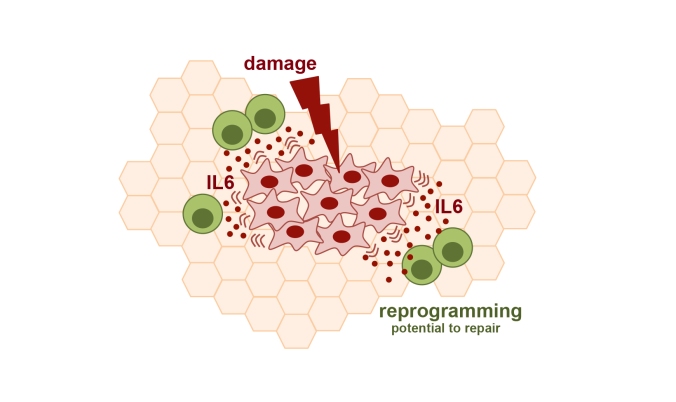
IL-6 released from damaged cells boosts reprogramming in surrounding cells with the potential for regenerative repair. (Image: Mosteiro et al. Science. 2016 Nov 25;354(6315))
Please note
It’s important to keep in mind that the end goal here is not to find ways to optimize the use of the Yamanaka genes for in vivo reprogramming efficiency in a clinical setting in people. Doing so would carry the dangerous risk of causing cancer. Instead, these results have revealed that senescent cells, through the action of IL-6, appear to stimulate the regeneration and repair of damaged or injured organs. A CNIO press release described how the scientists plan to apply these new insights:
“Having identified the essential role of IL6, … the team [is] now working on various pharmacological approaches to enhance the reprogramming efficiency, which could help to improve the regeneration of damaged tissue even in the absence of the Yamanaka genes. Improving the repairing capacity of tissues could have obvious implications for regenerative medicine, including the treatment of multiple pathologies and degenerative processes associated with ageing.”