 |
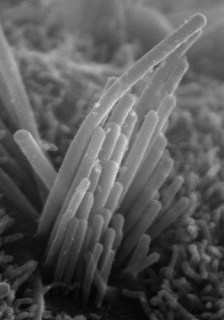
| Ear hair cell derived from embryonic stem cells | Stefan Heller, Stanford University School of Medicine |
This week researchers at the University of Sheffield in England used cells derived from embryonic stem cells to help restore hearing in gerbils. One note: the scientists weren’t driven by a concern for gerbil hearing. It just so happens that the rodents hear in roughly the same sound range as us humans and so their ears are a good model for human hearing.
This work, which was published in this week’s advanced online edition of Nature, differens from some other stem cell-related hearing projects in that it focused on the nerves that carry sound sensation from the ear to the brain. Most people with hearing loss have damage to the hair cells within the ear that first detect sound. This kind of damage can be aided in some cases by cochlear implants. But if the nerves that transmit signals from the cochlear implant to the brain are damaged then the person still can’t hear. That’s where this new technology would fit in.
A story in the Technology Review says:
The stem-cell treatment could eventually be combined with cochlear implants to give more deaf patients the ability to hear. But much more work would be required to bring this idea to fruition.
The story goes on to quote Stefan Hellar from Stanford University, who has a CIRM-funded project to generate the hair cells that first detect sound (those are his embryonic stem cell-derived ear hair cells above). Technology Review goes on:
While the study shows the potential of stem cells to replace auditory nerve fibers, says Stefan Heller, who studies hair-cell function and regeneration at the Stanford School of Medicine, the results will be difficult to translate to patients. “It is virtually impossible to diagnose a reduction of auditory nerve fibers in hearing-loss patients.”
Our hearing loss fact sheet has more information about stem cell research into hearing loss, including a few videos about the work we fund.
A.A.
Neural stem cells regenerate axons
SAN DIEGO — In a study at the University of California, San Diego and VA San Diego Healthcare, researchers were able to regenerate “an astonishing degree” of axonal growth at the site of severe spinal cord injury in rats. Their research revealed that early stage neurons have the ability to survive and extend axons to form new, functional neuronal relays across an injury site in the adult central nervous system (CNS).
The study also proved that at least some types of adult CNS axons can overcome a normally inhibitory growth environment to grow over long distances. Importantly, stem cells across species exhibit these properties. The work will be published in the journal Cell on Friday (Sept. 14).
The scientists embedded neural stem cells in a matrix of fibrin (a protein key to blood clotting that is already used in human neuron procedures), mixed with growth factors to form a gel. The gel was then applied to the injury site in rats with completely severed spinal cords.
“Using this method, after six weeks, the number of axons emerging from the injury site exceeded by 200-fold what had ever been seen before,” said Mark Tuszynski, M.D., Ph.D., professor in the UC San Diego Department of Neurosciences and director of the UC San Diego Center for Neural Repair, who headed the study. “The axons also grew 10 times the length of axons in any previous study and, importantly, the regeneration of these axons resulted in significant functional improvement.”
http://www.universityofcalifornia.edu/news/article/28333
The use of embryonic stem cells, -i.e. originally taken from embryonic human beings who were killed in the process- is a disgusting and unscrupulous abuse of medicine worthy of Mengele. The end does not justify the means .
Human Embryonic Stem Cells do not have to be harmed or killed to extract the stem cell through Advanced Cell Technologies Blastomere process. They are the only company to have an FDA approved clinical trial. Cirm REJECTED their funding and FORCED them almost out of business. It is said and well discussed that there was a conflict of interest with the reviewer that REJECTED their funding.
Its actually funny is you read the complaint.
Chairman Klein response to Advanced Cell was basically sorry we need to go to lunch!
Its Public Info – http://www.cirm.ca.gov/files/transcripts/pdf/2008/08-13-08.pdf
MR. KESSLER: MY NAME IS STEVEN KESSLER.
I'M WITH ANOTHER FOR-PROFIT ORGANIZATION, ADVANCE
CELL TECHNOLOGY. WE PUT IN A NUMBER OF GRANT
PROPOSALS WHEN WE WERE FINALLY ALLOWED TO SUBMIT
PROPOSALS. AND, OF COURSE, THEY WERE NOT
RECOMMENDED FOR FUNDING.
I'D LIKE TO POINT OUT TO THE BOARD THAT
IT'S MY UNDERSTANDING THAT FOR THE NEW CELL LINES
AWARDS, THAT THERE WERE 58 PROPOSALS OVERALL, THAT
12 CAME FROM FOR-PROFIT ORGANIZATIONS, NONE OF THOSE
WERE RECOMMENDED FOR FUNDING, COMPARED TO PRESUMABLY
18 OF 38 FROM NOT-FOR-PROFIT ORGANIZATIONS. TO ME,
THAT'S CLEAR EVIDENCE OF BIAS. I'VE HAD — WE
FORMALLY APPEALED THIS ON JUNE 26TH. THE APPEAL, I
WAS THE PI ON ONE OF THEM, WAS TWO AND A HALF PAGES
DESCRIBING FOR CIRM WHAT A CONFLICT OF INTEREST IS
FROM A BUSINESS PERSPECTIVE, AND ANOTHER SEVEN AND A
HALF PAGES SPECIFICALLY REBUTTING POINT BY POINT ALL
THE REVIEWERS' CRITICISMS.
I DON'T SEE HOW WE CAN DO THIS. WE CAN
CERTAINLY CONDENSE THE LETTER, BUT WHAT WE DID WAS
CITE THE REVIEWERS' COMMENTS AND WHAT OUR PROPOSAL
ACTUALLY STATED THAT FACTUALLY CONTRADICTED THAT.
YOU KNOW, IT WOULD BE MORE DIFFICULT, I THINK, FOR
THE BOARD TO UNDERSTAND WHAT ACTUALLY TOOK PLACE IF
WE DIDN'T DO IT IN THAT TYPE OF A SEQUENCE.
BUT JUST TO COME BACK TO THE CONFLICT OF
INTEREST ISSUES, I'VE HAD DISCUSSIONS WITH CIRM
STAFF JUST THIS WEEK FOLLOWING UP CITING CIRM'S OWN
CONFLICT OF INTEREST POLICY TO THEM ON SPECIFIC
ISSUES. ONE ISSUE WAS, YOU KNOW, IF A GRANT
REVIEWER HAS A FINANCIAL RELATIONSHIP WITH COMPANY
X, YOU KNOW, THAT IS, HE'S RECEIVING FUNDING FROM
THAT ORGANIZATION OR HE'S EXPECTING ROYALTY INCOME
FROM SOME COMPANY BY VIRTUE OF HAVING LICENSED
TECHNOLOGY TO THAT COMPANY. AND THAT REVIEWER IS
SITTING IN ON REVIEWS FROM OTHER FOR-PROFIT
ORGANIZATIONS, COMPANIES A, B, OR C, AND DOESN'T
RECOMMEND THOSE FOR FUNDING. TO US, FROM A BUSINESS
PERSPECTIVE, THAT'S A CONFLICT OF INTEREST.
THIS IS A BIT DIFFERENT FROM THE SITUATION
IN ACADEMIA WHERE PEOPLE QUIBBLE ABOUT
TECHNICALITIES ON PAPERS. YOU KNOW, FOR-PROFIT
ORGANIZATIONS, THEY INVEST MILLIONS OF DOLLARS
BUILDING UP THEIR PATENT ESTATE BECAUSE THEY WON'T
PROCEED DOWN THE DEVELOPMENT PATH, WHICH INCURS, YOU
KNOW, TENS OF MILLIONS OF DOLLARS IN EXPENSES,
UNLESS THEY HAVE A CLEAR PATENT PATH.
SO IT IS REALLY A SERIOUS ISSUE IF, YOU
KNOW, SOMEBODY, SOME REVIEWER WITH CONFLICTS, AND WE
CITED NUMEROUS INSTANCES, IN OUR VIEW THERE'S NO
REASON TO RECITE THOSE RIGHT NOW, WHERE, YOU KNOW, A
REVIEWER WOULD HAVE EVERY INCENTIVE TO HELP IMPEDE
THE COMPETITION FOR THE COMPANY THAT HE HAS A
RELATIONSHIP WITH.
CHAIRMAN KLEIN: WELL, I VERY MUCH
APPRECIATE THOSE COMMENTS. WE DO HAVE AN EXISTING
SEPARATE POLICY FOR CONFLICTS. WE TREAT THEM VERY
SERIOUSLY. WE HAVE A SEPARATE POLICY IN PLACE FOR
RE-REVIEW IN THE EVENT OF CONFLICTS. AND THE STAFF
DOES A THOUGHTFUL ANALYSIS. THEY HAVE GONE THROUGH
AND FOUND A CONFLICT IN A PARTICULAR — POTENTIAL —
EVEN IF THEY FIND A POTENTIAL FOR A CONFLICT, THEY
LOOK AT POTENTIALLY RE-REVIEWING IT. THEY'RE TRYING
TO ERR ON THE SIDE OF EQUITY AND JUSTICE HERE.
MR. KESSLER: I REALIZE THAT. AND I WAS
TOLD THAT THE WAY CIRM INTERPRETS ITS OWN CONFLICT
OF INTEREST POLICY, THE EXAMPLE I GAVE YOU WAS NOT A
CONFLICT OF INTEREST. SO —
CHAIRMAN KLEIN: OKAY. SO WE'LL HAVE THE
OPPORTUNITY BETWEEN NOW AND THE NEXT SESSION TO LOOK
AT THAT AS WELL, BUT WE APPRECIATE YOUR COMMENTS.
THANK YOU VERY MUCH.
I'D LIKE TO, WITH THE BENEFIT OF VERY
THOUGHTFUL DISCUSSION HERE, TO MOVE TO THE NEXT
115,000 abortions a day,42 mllion abortions a year in the world.go for it.
The cells used in this study are embryonic stem cells, which were generated from 4-5 day old embryos from IVF clinics. It is a common misconception that embryonic stem cells come from aborted fetuses. In fact, embryonic stem cells are a fleeting cell type that only exists in several day old embryos.
The embryos used to create embryonic stem cells in this study were donated by couples who had completed their families through IVF. They can choose to simply discard the extra embryos, donate those embryos to medical research, or donate those embryos to another family. In this case, the couples chose to donate their extra embryos to medical research.
My name is David. I have had nerve damage on my ears all my life since birth. I would like to know if I could be a candidate for stem nerve replacement in my ears. Or just one ear.. I know I would be a good candidate would you please let me know if there are any clinical trials that I may try. I know the government has a lot of universities to do a lot of research. They have come a long ways I’ve had this for 60 years. I just would like to be able to hear one day. Thank you very much for your time.
My wife suffered a hearing loss about two years now following treatment from hospital. How can my wife receive this research trial on stem cell and implant to make her hear again?
Dear Mr. Akpan, I am so sorry to hear about our wife’s hearing loss. That must make life very difficult for her. I am afraid I don’t know of any clinical trials underway right now that are addressing that problem and that would be able to help your wife. I hope that in the not too distant future we will have treatments to help people with hearing loss, but that may be some years away.
My husband suffered a hearing loss on both ears due to nerve damage . I ask to know which hearing device will be suitable for him and how much the procedure will cost
Dear Zuzeka, thanks for the comment on our blog unfortunately we only work with stem cells. You would need to consult a hearing specialist to find out more about hearing devices.