The California Institute for Regenerative Medicine (CIRM) recently announced funding a series of projects spanning the earliest stages of research through to clinical trials. One of those projects was an $8 million award to Matthew Pollman, MD, MS, Senior Vice President of Clinical Development at Tenaya Therapeutics, to support a clinical trial focused on treating a rare heart condition called Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC).
CIRM funds two levels of clinical awards: CLIN1 awards support the stages of testing and verifying a potential therapy prior to starting clinical trials; CLIN2 awards support projects that are already being tested in humans. The award to Tenaya Therapeutics is a CLIN2 and will support a potential therapy that is already in clinical trials. This is one of over 110 clinical trials that CIRM supports.
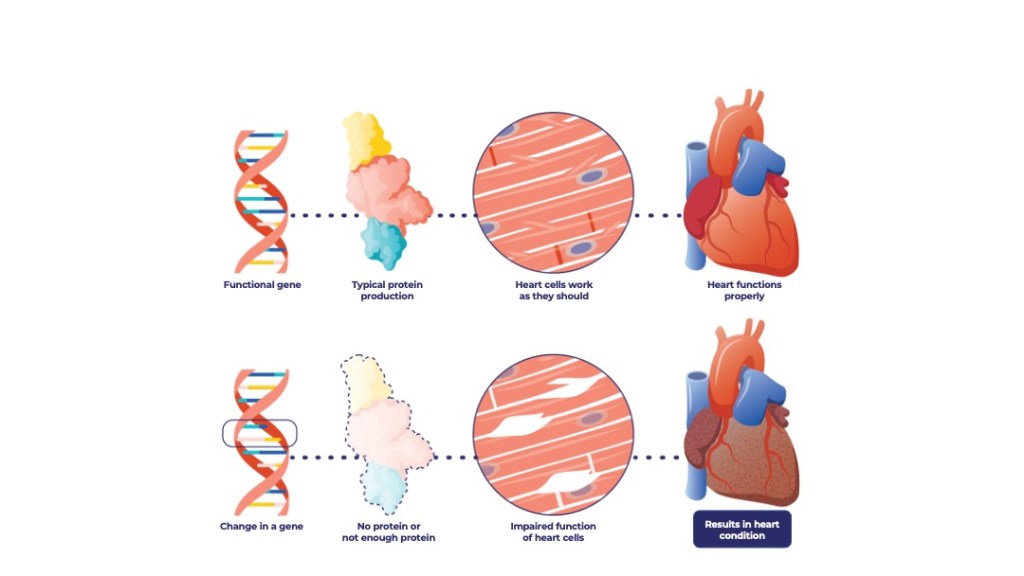
ARVC is frequently caused by a mutation in the plakophilin-2 (PKP2) gene, which makes a protein that is essential for proper heart function. People with the mutation generally begin experiencing symptoms as young adults and have a high risk of life-threatening heart conditions such as ventricular arrhythmias, sudden cardiac death, and progression to heart failure. There is no treatment for the condition, which affects 70,000 people in the U.S.
Tenaya’s clinical trial is testing a gene therapy approach that would replace the mutated gene in heart muscle with a normal version of the gene. If successful, the new gene would produce a functional copy of the protein and potentially restore healthy heart function and slow or reverse disease progression.
“We are honored to receive this grant from the California Institute for Regenerative Medicine, which will support our ongoing RIDGE-1 clinical trial of TN-401, a potential best-in-class gene therapy intended to address symptomatic patients carrying PKP2 gene mutations, the most frequent genetic cause of arrhythmogenic right ventricular cardiomyopathy,” said Dr. Pollman. “Our goal is to harness the power of gene therapy to correct the underlying cause of this severe and progressive disease, offering new hope for an improved quality of life to those the individuals and families affected by this debilitating condition.”
Written by guest contributor Amy Adams